Back هقزاافلوئورواتان AZB Hexafluoroetà Catalan Hexafluorethan Czech Hexafluorethan German هگزافلوئورواتان Persian Hexafluoroéthane French हेक्साफ्लोरोइथेन Hindi Hexafluorethaan Dutch Hexafluoroetano Portuguese Heksafluoroetan Serbo-Croatian
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hexafluoroethane | |||
| Other names
Carbon hexafluoride, 1,1,1,2,2,2-Hexafluoroethane, Perfluoroethane, Ethforane, Halocarbon 116, PFC-116, CFC-116, R-116, Arcton 116, Halon 2600, UN 2193
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.855 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2193 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
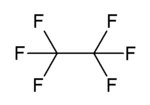
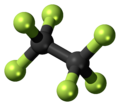
| C2F6 | |||
| Molar mass | 138.01 g.mol−1 | ||
| Appearance | Colorless odorless gas | ||
| Density | 5.734 kg.m−3 at 24 °C | ||
| Melting point | −100.6 °C (−149.1 °F; 172.6 K) | ||
| Boiling point | −78.2 °C (−108.8 °F; 195.0 K) | ||
| 0.0015% | |||
| log P | 2 | ||
Henry's law
constant (kH) |
0.000058 mol.kg−1.bar−1 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Supplementary data page | |||
| Hexafluoroethane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hexafluoroethane is the perfluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in methanol. It is an extremely potent and long-lived greenhouse gas.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search